Anti-biology: a thesis praeludium
Will the biggest biotech breakthroughs actually come from physics?
I declare myself guilty. I constantly write things like “It’s time to GROW everything!!!”, have helped biotech cos develop engaging branding, and still get excited about people like Eric Schmidt retweeting my article on his biotech bet. In a few words, I’m ultra-bullish for bio.
Ironically, attending the Global SynbioBeta conference this year (such a “biosimp” like me couldn't miss it), was an opportunity to seriously ask where my glowing dinosaur might be. Technologies like microfluidics, cell culture, and biosensors have been here for decades and yet most haven’t realized our overarching vision of them, like 3D printed organs or cheap diagnostics for all. A-la Theranos, except we want them for real.
It was he who’s seen as one of the fathers of synthetic biology; he whose last name sounds quite religious, who shared a rather bio-unorthodox idea that’s been living rent-free in my mind for months: the biggest breakthroughs in biotech will actually come from physics1–George Church.
X-ray crystallography, MRIs, mass spectrometry, Cryo-Electron Microscopy, optical tweezers, and even the microwave to make gels are all examples of enabling tools that, throughout time, have morphed and migrated, from unique research projects in the physics department to ubiquitous, essential, equipment in the biology department.
For this third piece of Pillars of the Bioeconomy, I unfortunately won’t show you glowing dinosaurs. The companies I’ll tell you about are essentially building better tools for biotechnologists. From bioelectronics and the neglected problem of DNA extraction, to 3D-printed bioreactors with 7x’d efficiency that you’ll love to have on your desktop as a drug developer.
This article has been fermenting in my drafts for 3 months now. My recent experiences in the lab, talking to really interesting bio people and reading more about biophysics have turned it into “only” a prelude, a LatAm special edition, to yet another piece that I earnestly cannot wait to show you. Let’s dive into some GRIDX biotechs now!
Will your bioreactors meat the demand?
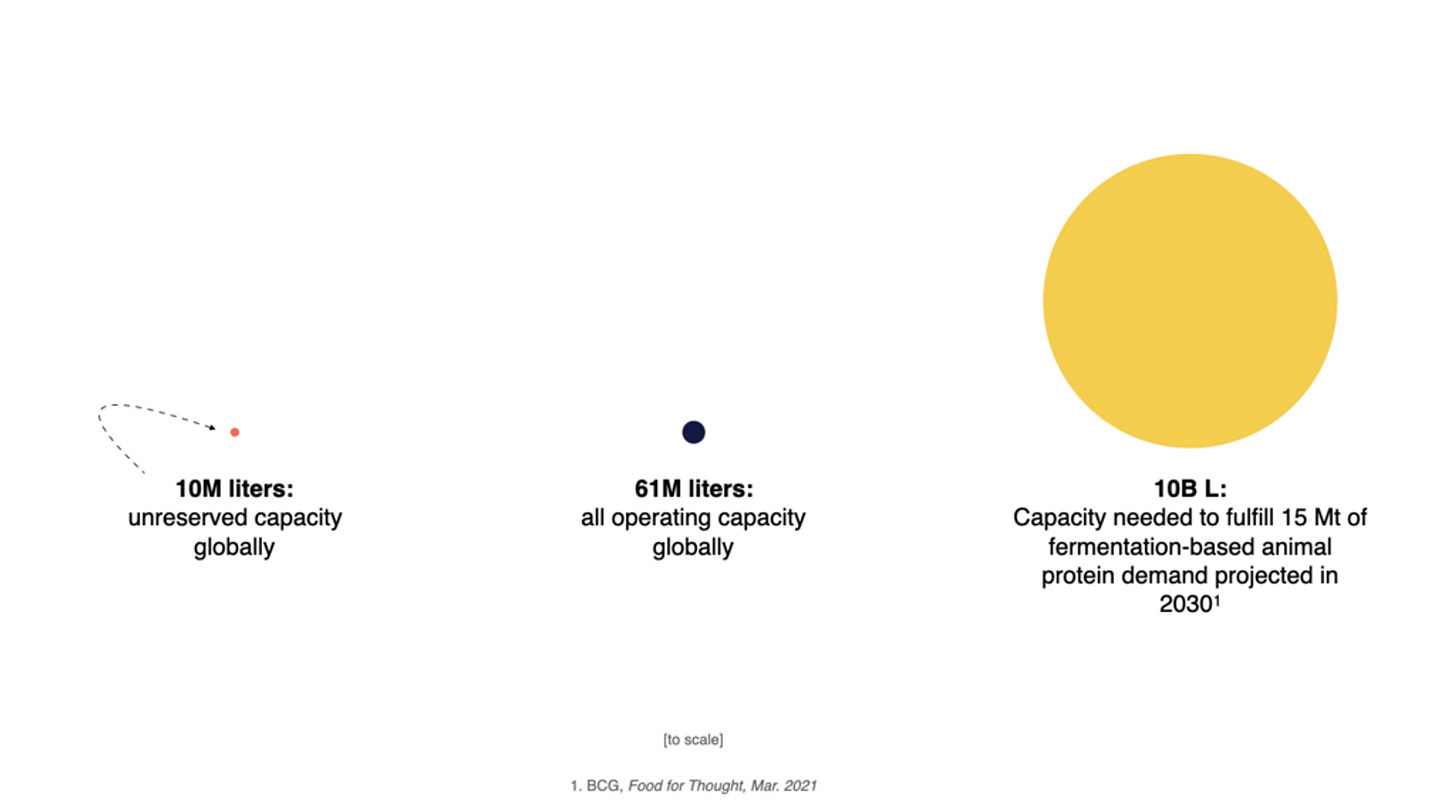
The image below says it all. Producing 15 megatons of animal protein through microbes would require 100x more than the world’s current capacity supply. For context, that’s less than 10% of the estimated global meat demand by 2030. Producing that much cell-based meat would require 4,000 thousand facilities with 10x the output of current ones.
The COVID-19 crisis exposed the scarcity of manufacturing infrastructure for the production of vaccines and antibodies. The problem is not only the number of bioreactors but in the limited capabilities that each of them has and the high manufacturing cost that these devices still represent.
I’d really appreciate someone writing a detailed cost breakdown of a standard stirred tank bioreactor. For now, we can analyze some of its main parts to get a gist of it for now:
Vessel: usually, the highest-capacity ones are made out of steel while glass and plastic bags can be used for smaller bioreactors.
Agitation system: its mission is to transport nutrients to, and waste products away, from the cells. Impellers provide agitation, heat transfer, and assist the spargers in aeration by breaking down and mixing bubbles throughout the system. Operating through magnetic stirrers or gas-inducing impellers is more energy-intensive.
Aeration system: dissolved oxygen concentration can be a limiting factor for cell growth. Each bioreactor has its own aeration capacity which will determine the need for microspargers that transfer oxygen-rich bubbles. This system also includes pressure controllers, gas filters, and valves.
Sensors and controls: temperature, pH, oxygen, cell mass, essential nutrient levels, and product concentration.
Founded in 2014, Stämm’s mission is to make biomanufacturing easy, scalable, and repeatable; to decentralize bioprocesses and democratize access to biotechnology products.
Meet the Bioprocessor: a whole biotech facility downsized by 200x into an all-in-one, plug & play desktop unit. Inside it, you will find that cells flow in unidirectional laminar flow through crystallographic vasculature while the growth medium is continuously sterilized through Pulse Electric Field technology, with microfluidic devices controlling aeration and agitation. All of this, cased inside a 3D printed vessel made out of biocompatible materials.
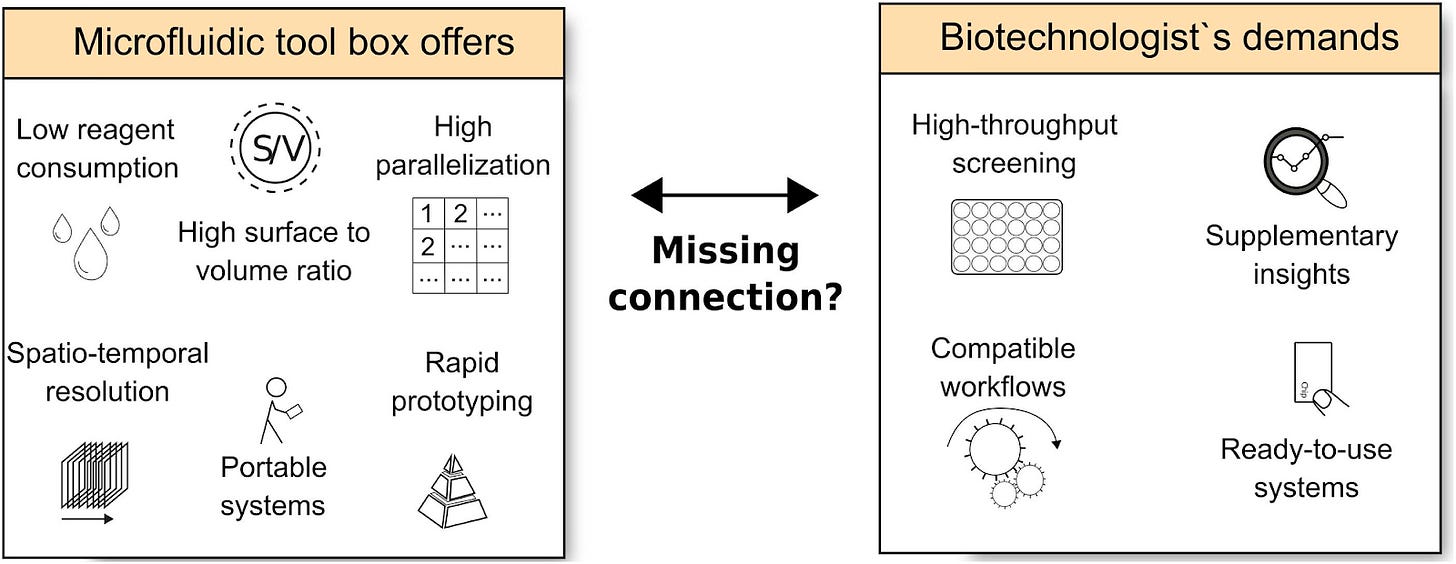
Microfluidics had been mostly used in inkjet printheads since the 80s, and just more recently in DNA chips and lab-on-a-chip tech too. Some of their advantages include low reagent consumption, high surface volume that allows for fast mass and heat transport, high spacio-temporal resolution to study even single cells over time, rapid prototyping in custom-made based PDMS soft lithography, and portability.
So where are all our labs-on-chips at? One objection to using microfluidics in biology is actually in the micro part. Usually, working only with a few hundred or few thousand cells is not statistically relevant. From a naïve point of view, throwing a technology “that can do it all for anyone” to everyone sounds like indefinite optimism. Covering a large part of a niche, a specific market might be a better plan for world conquering.
Following that thinking, Stämm’s bioreactors can currently reach an output of about 30 liters. However, this design is already >7x more productive than any other. Thus, Stämm is not looking to make 5000-liter bioreactors but to completely replace them.
I’ve contextualized bioreactors much in the context that I’m most familiar with, which is cellular agriculture. However, it makes total sense that Stämm is starting to sell their products in the biopharma space where margins are higher and the markets are larger.
Stämm is not your conventional biotech that recently raised 17 million dollars. This isa a team of builders who built their own 3D printer for their microfluidic devices and who are on a mission to grow a network of Bioprocessors worldwide.
Also, this is not one more of my bio-bullish articles. For incredible Stämm is—and oh boy I’d be thrilled to have a Bioprocessor on my desk—they’re not gonna make it alone. No one is. The global bioeconomy needs such scales that we’re gonna need way more great startups building just as they are. I hope you decide to take that leap yourself.
A neglected biotechnology
In previous articles I’ve talked about DNA sequencing and editing as enabling biotechnologies for the bioeconomy; the things that are making it exponentially easier and cheaper to build with biology (i.e., the transistors of biotech). Indeed, the global synthetic biology market size was valued at USD 13.09 billion in 2022 and you won’t guess what biotech sector had the highest market share.
PCR, 27.29%. The technology you most likely got to know only because of COVID, is another crucial biotechnology with applications that range from forensic research to pretty much anything that deals with genetic material in a synthetic biology product.
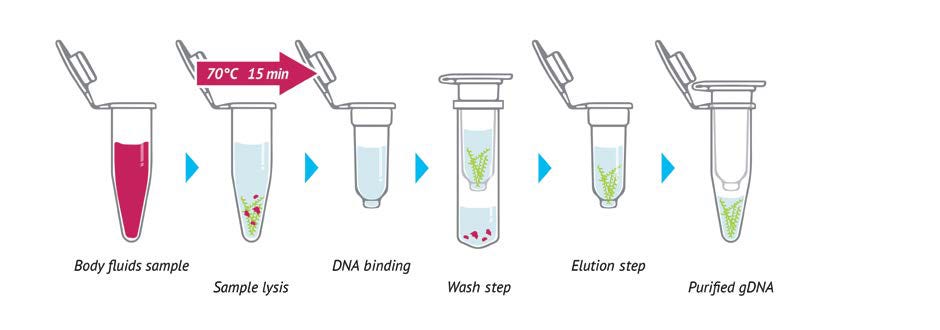
But that is not the neglected technology I’m talking about. When we want to do PCR or sequencing of DNA in a cell (the sample is not readily available as forensic samples, for instance), we need to… that’s right! We need to extract it. And it sucks.
It sucks because, for the most part, it can only be done inside a lab. While the cost in sequencing doesn’t stop dropping and we have things like the minION, extraction has become stagnant. When we think of a $100 human genome coming to market soon, is it sound to think that extraction would add up to 25% of that to the total?
Now, let’s think you’re an awesome company like BaseCamp Research that’s taking samples in wild places, or a researcher who just got a blood sample and the costs of storing it for analysis are high OR simply a business in the 21st century that works with genetic material (remember the PCR market share). You don’t want to wait to be at the lab, some samples you have are time-sensitive so you can’t risk losing them. And being such a routine task, wouldn't every bio and non-biotech business want to significantly reduce their expenses through cheaper DNA extraction methods? Where’s our lab-in-a-box? Where’s the minION of extraction?
NanoJump is here to get that done. Their portable device facilitates the extraction of the genome anywhere, by anyone and from any cell. Inside it are microchannels that contain chemical and nanotech solutions that inactivate microorganisms (such as viruses and bacteria) and extract genetic material simply and safely, anywhere and from any sample. They understand that the study of DNA and RNA will continue to impact the value chain of multiple industries, not exclusively biotech companies.
I genuinely hate that I can’t tell you much about them yet as they’re kind of in stealth mode but keep your eye on them if this sounds interesting.
Where’s my BioComputer?!
The question that many of the biotech visionaries of our era are asking, including myself. Though I’m by no means qualified to speak about the true essence of Apple, I’ll suggest that a key phrase could be “To enable anyone to create anything”. Not only the CS college nerds, not only IBM executives. But your 37-year-old hippie aunt who’s a great painter, your best friend who’s a compulsive gamer, your high school vice principal who used to use a strange thing called BlackBerry before he got an iPhone. Apple believed that extraordinariness can arise from the ordinary. They knew that anyone can cook, so they created tools that enabled and inspired the next generation of chefs.
Creation is not only an artistic affair but an everyday act that can extend from medicine to botanics. So the question remains: who is enabling anyone to create with biology and what are they creating? I hate to disappoint but I don’t have a definite answer to that right now. However, if we guide ourselves through the same line of enabling, we might find some interesting proposals.
Our global medical system is literally dying for creators. As a latina, it’s staggering to even think that 70% of poor people in the region–that’s 140 million people–don’t have access to basic healthcare service. That despite our seemingly advanced biotechnologies, more than 3 million people still die each year due to cancer and cardiovascular diseases in Latin America.
Of course, when it comes to a place like this, you can’t blame it all on technology costs. There’s corruption, there’s misallocation of capital, lack of health education, and a bunch of other social factors that directly or indirectly explain all these deaths.
Still, my mission here is to think of what needs to be true in order for technology to help solve these problems. So we already know that the best “cure” for most of these diseases lies in early diagnosis. In different types of cancer, survival rates can go up to 89% when detected early, that cardiovascular diseases benefit from blood tests too, and even when we can all remember how crucial early detection was for the first strains of COVID as an infectious disease.
It's two things that all of these examples have in common: 1) they’re using some sort of biosensor at some point in the process; 2) our current biosensors are making most of these diagnostics expensive, making healthcare a centralized and costly privilege instead of an immediately accessible right. How? Let’s dig deeper…
To start, a biosensor is a device that measures the concentration of a chemical or biological analyte (i.e. compound of your interest) and lets you know through some sort of visual interface. It does this by binding a receptor to the analyte, which emits a signal that is recognized and translated by a transducer. An electronic component detects this translated energy and displays it.
Analyte: substance of interest (e.g. glucose).
Bioreceptor: molecule that specifically recognises the analyte (e.g. enzymes, cells, aptamers, DNA and antibodies) and emits a signal generation in the form of light, heat, pH, charge or mass change, etc.
Transducer: element that converts one form of energy (in very little amounts) into another. In this case, into a measurable signal, usually optical or electrical, proportional to the amount of analyte–bioreceptor interactions.
Electronics: amplifies or converts signals from analogue into digital, that are then quantified by the display unit of the biosensor.
Display: User interpretation system such as the liquid crystal display of a computer or a direct printer that generates numbers or understandable curves.
Aside from the COVID test, perhaps the most popular examples of these so-called biosensors are the glucometer that uses the glucose oxidase or dehydrogenase enzyme to detect the concentration of glucose in blood, and the pregnancy test that operates through monoclonal antibodies that attach to the hCG hormone which is only present after fertilization of the ovule.
Evidently, all of the previous biosensors have scaled to enable millions, perhaps now billions of people to get tested. Wouldn't it be sad though, if our computers were only made to do a single thing and you had to buy a different computer for each app you want to use? The magic of the first iPhone was making it a thing of the past to use different devices for different purposes.

It’s also clear that our existing biosensors can perform only a low number of, and similar, diagnostic assays. As we advance through the stepping stones towards the legendary biocomputer, we face some problems…
See, biosensors are similar to drugs in that development takes lots of money and several years. However, it’s actually worse with biosensors because, unlike in the pharmaceutical industry, there’s no specific methodology to discover new receptors. If you remember, receptors are the parts that recognize the analyte, so they should be highly specific, stable under storage conditions, and immobilized.
After all that context, it’s time to tell you about two biotechs that are developing healthcare devices that are getting us a little closer to the iPhone experience.
Aplife Biotech, the bioelectronics company, has created AptiveX: the world’s first digital probe discovery platform. Through large combinatorial libraries of synthetic DNA that are embedded on CMOS-type semiconductors, they enable biosensor companies to quickly, accurately, and cost-effectively discover new receptors.
They started in 2020 as a merger between business people who are set to bring great products into the hands of millions, and scientists with a long experience of DNA research who have a deep desire to overcome the restraints on digital diagnostic devices. What’s more, these probes are not limited to point of care applications but will also become a useful tool in precision agriculture, DNA data storage, lab-on-a-chip developments, and epidemiology.
If the ultimate dream is an all-in-one biosensor device, Gisens Biotech is up for the deal. Through nanotech and multiprocessor electronics, they’ve created the nanoLab: a diagnostic platform that’s the same size and weight as a smartphone AND connects to your smartphone through bluetooth, into the Gisens app. Plus, it requires no chemical reagents or calibration. It’s all about the nano…
Nanostructure field effect transistors (nano-FET)–electronic devices used to detect and measure the concentration of specific biological molecules. Essentially, they work through receptors (like those that Aplife helps design) which experience a change in potential when attaching to the analyte.
Through two drops of urine, blood, or saliva, the nanoLab can test for hundreds of biomarkers in just 5 minutes, at half the price of conventional tests and on-par with the sensitivity of PCR tests and ELISA methods. The versatility of their platform was proved when, after receiving a $100k prize from MIT and ITBA in 2020, Gisens was rapidly able to adapt their platform to test for COVID too.
They’ve been operating for more than 4 years now and are currently based in Berkeley, Gisens is on a mission to change point of care diagnostics, for real.
Some closing thoughts
After this brief exploration, I noticed how technologies with clear value propositions like microfluidics can thrive when focused towards precise applications, that neglected areas of opportunity like DNA extraction can have huge markets, and that understanding the major cost-drivers of inventions like biosensors might help to take them into the hands of millions, for real.
As we approach the future that our predecessors could only dream of, I think it’s important to remember that time doesn’t equal progress and that that progress doesn’t always look as we imagine. Our flying cars and teleporting machines are rather zoom rooms and social media. Does this mean that our ambition and grit towards greatness was diminished or that we just found a different way to do things?
It might be equivocal to compare biotechnology to technology in any way (yeah, like I just did) or bullishly claim we are where others were in the 60s. After all, we didn’t get transistors and iPhones by analogy, did we? As we grow the biotech industry, it might be useful to keep with us first principles thinking, an infinite imagination, and an atom or two of physics.
Thanks for reading. If you’re onto something just as cool or even cooler than the companies in this article, don’t hesitate to reach out to me or GRIDX.








how do you make all of your substack post image covers? They're always quite intriguing and well done!!