Pillars for a global bioeconomy #2: Edit
History, markets and future of synthetic biology in LatAm and the world
Look at your surroundings. Chances are that you, like me, are surrounded by lots of plastic goods (yes, clothes count too). If you recall the last meal you had, chances are that it contained some sort of synthetic dye or unhealthy amount of sugar.
While many advocate for reducing consumption in a world of scarcity, synthetic biology promises a world of abundance where we continue to grow our favorite natural goods through more sustainable processes. While we live in a world of “general” medicine and physicians today, tomorrow’s precision medicine will attack disease at each of our cells’ nucleus.
As we advance through our quest for biochemical literacy, we’re becoming able to write, edit, and replicate biomolecules that meet several industries’ standards. Over the next 50 years, we will be using these biotechnologies to reverse-engineer 10x more sustainable products for both human and planetary health.
Thanks to GRIDX, the largest biotech company builder in Latam, for supporting this post and the biotech startups that are changing the game in our region by using and advancing 3 pillar biotechnologies: sequencing, synthesis & editing, and bioinformatics.
Write, edit, grow
In our last edition of Pillars of the Bioeconomy, we covered how to read biological molecules like DNA in order to understand health and disease. Chronically, this issue is about writing and editing those molecules, in humans and other living creatures.
DNA synthesis
In this context, you can refer to DNA as oligonucleotide. These are relatively short fractions of DNA or RNA made up from building blocks called phosphoramidites. The latter are basically nucleotides that are protected with some caps so they don’t react with anything else before adding another block.
The blocks are added sequentially as the designer specifies. The challenge here is that the longer the chain gets (being 200 nucleotides the current limit), the more errors we get which is the big elephant in the room when it comes to DNA synthesis and synthetic biology overall.
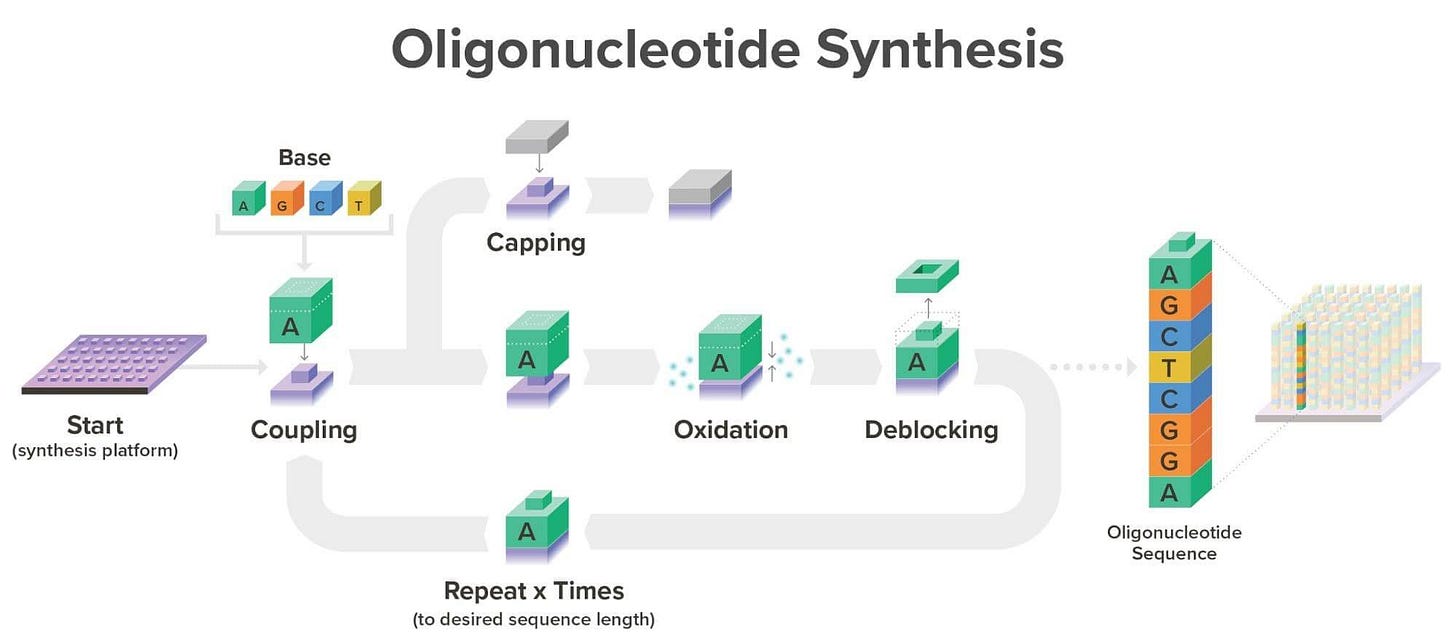
Big synthesis companies like Twist Bioscience are working on this challenge. They use a silicon “chip” with thousands of individual reaction wells which allow them to produce nearly 10,000x more genes at 1,000,000x lower reaction volume than traditional DNA synthesis—now that is moonshot thinking.
Gene editing
Let’s jump into editing! As a reader outside of the biotech space, I wouldn’t be surprised if you’d already heard of CRISPR or gene editing in general. Even though we’ve been editing DNA artificially (through radiation) since the 1920s, it was only until the 70s that we started using more precise, biomolecular approaches such as recombinant enzymes and Zinc Finger Nucleases.
CRISPR in particular was discovered by studying how bacteria defend themselves against viruses; a great example of how basic science and curiosity can take us places. Recently, Jennifer Doudna and Emmanuel Charpentier won the Nobel Prize for further developing this mechanism into a technology that can be used for therapeutics and diagnostics.
Briefly, you can think of CRISPR as a machine consisting of a GPS (called gRNA), scissors (called Cas enzyme), and a spare tire. Next time you want to replace a sequence of DNA, all you need to do is:
Design a gRNA which is equal to the sequence that you want to replace
Choose a Cas enzyme to cut the undesired sequence
Design a new sequence
Put them together into a delivery system like a “good” virus or lipid
Deliver it
As always, this is easier said than done so please don’t take this as serious advice :)
As groundbreaking as CRISPR has been, it’s also just the beginning. Many different types of Cas enzymes have been found in bacteria. Technically, they differ in the kind of “GPS” they need, how they cut nucleotides and thus how these are repaired inside the cell, whether they targets RNA or DNA, and the length of the sequence of the enzyme and the gRNA which in turn determine how many genes we can edit at a time (how multiplexed the system can be).
Practically, the difference also lies in the application that each enzyme can be given. Cas13 is quite an interesting example of a CRISPR enzyme whose natural features can be harnessed and further engineered to achieve low-cost and fast diagnosis (e.g. in zika and SARS-CoV2).
There have been many more developments along this vertical, including base editing and prime editing which convert single DNA bases without the need to even cut DNA! With over 90% of human diseases being caused by changes in a single nucleotide (officially called SNPs), these new “breeds” of CRISPR could be quite promising.
10 GRIDX startups
Okay, but how about writing new DNA sequences? What if we could engineer novel organisms all-together? If we can sequence and synthesize any DNA sequence, if we can analyze and design new sequences…
Does that mean we can edit fungi to produce food dyes that are better for your food and your health? How about creating RNA molecules that attack pests in a non-toxic and safe way to increase crop survival by 40%? Or saving 17 lives by making pig organs compatible for transplant in humans? Vaccines for fish that to prevent $2 billion in losses in the pisciculture industry?
These are all products that 10 GRIDX startups are developing. Many of them use a combination of pillar biotechnologies, like sequencing, synthesis, bioinformatics, and fermentation. If the solutions or the market sound interesting, you’ll want to keep reading.
Feedvax
How often do you think about the ocean as a place where some of the world’s biggest problems? How about thinking of it as a growing industry? Bad news and good news: it’s both.
Seafood accounts for 17% of the world’s protein, the fish & seafood industry is $2.1 bn in 2023, and still it’s easy to forget about the ocean. With increasing demand, aquaculture (or fish farming) is now meeting 50% of the world’s demand for fish. The big problem? Fish disease.
If you, like me, hadn’t even considered the possibility of fish getting sick, I shall share that this problem causes losses of up to 30% of the production. Perhaps you’re wonder: oh why can’t these fish farmers just give them their pills?!
Before entering fish pills, we shall know why this is something that people want and doesn’t hurt anyone. In fact, current vaccines are not only hard to deliver but are also causing the same problem that in human disease: antibiotic resistance.
That said, oral vaccines have always had both technical and commercial challenges, namely keeping antigens from reaching immune induction sites before they degrade and scaling the production of virus antigens.
Feedvax is a Hawaii-based startup with the vision of transforming animal production, starting with oral vaccines for fish. They designed an easy-to-use oral vaccine that goes in the feed pellet (wouldn’t that be great to have for humans and other animals too?!).
Their first product, a vaccine that tackles Streptococcosis in Tilapia, has already achieved +80% survival rate, demonstrating that Feedvax’s products are non-toxic and can even enhance the performance in healthy fish.
This reminds me of one of the mindsets for bioengineering startups I talked about in a previous post: platforms. Feedvax is not only growing pills for fish: they’re building a platform for easier vaccine delivery in the animals from whom we feed.
Yes—even though lab-grown meat is already here, most of us will keep feeding off of animals whom we’d better make sure are healthy :)
Michroma
If you’re just getting into biotech, here’s what you need to know: microbes are the machines powering the transition of dozens of industries into biological production of goods. Textiles, cancer sensors, fertilizers, and protein, are only some examples of products that microorganisms can grow: we’re living, as Elliot Hershberg would point in an insightful essay, the biologization of industries.
One of these is the food colorants, 2-billion-dollar industry. Somehow hidden in my last meal, were red dye 40 and yellow 5, two of the most common ingredients, which have also been linked to hyperactivity, certain types of cancer, and allergies.
Though the FDA hasn’t changed the regulations for these ingredients, the growing concerns are clear: no one wants to get weird things inside their bodies but no one wants to quit their favorite foods either. Further, current natural alternatives find it hard to meet industry standards like pH and temperature stability (i.e., some of these dyes wouldn’t work on that delicious cake you bake).
Since 2019, Michroma has been building a fungi-powered platform to produce next-gen natural ingredients (e.g. flavors, fragances, and colorants) in a sustainable, cost effective and scalable way.
With 90% of the food market running being painted on warm colors, their first product was RED+: the vibrant, pH and temperature stable, cost-effective, vegan, kosher, halal, and zero-residual taste, 10x better colorant that can be applied to candy, dairy, plant-based products and beyond.
Why, out of the 1 trillion+ number of microbial species on Earth, did they choose filamentous fungi? Ricky (cofounder and CEO of Michroma) explains that even though they’re actually harder to edit and ferment (compared to yeast), fungi have significantly greater yields, better secretion system, and higher titer.
Further, these are not ultra-novel biofactories: over 50% of commercially available proteins are produced by fungi!! The challenge is going from producing detergent only to food ingredients.
As you might imagine, that’s when gene editing comes in handy. We can hack the fungi system a bit, reducing the degradation of the desired protein, improve protein transport, or giving fungi instructions to produce a combination of the proteins it already has with ones we design or ones from other organisms.
Ricky was already a serial entrepreneur before he joined efforts with his co-founder Dr. Mauricio Braia, an expert in industrial biotechnology and the CSO genius mind behind Michroma’s products.
Together with their marvelous team, they’ve very recently raised their seed round, led by Supply Change Capital led it with participation from Be8 Ventures, CJ CheilJedang and others.
Syocin
What does cancer have to do with pests? I think we would all agree that, despite being a solution, chemotherapy is an archaic way to treat cancer. I see it as throwing a bomb at innocent cells just to kill the bad guys.
Well, the news is that we’re still doing that with plants! By this point, you won’t be surprised to know that our current agriculture OS suffers from lots of leakages in different areas. The FAO estimates that 20-40% of global crop production are lost annually to pests, costing the global economy around US $220 billion.
36 of those billion are due to bacterial diseases. Current solutions? Copper-based ingredients and antibiotics that cause resistance, hurt the soil microbiome, and pose a threat on human health that stands along the food production chain.
Something I love about synthetic biology is that it’s all about deploying solutions with and for nature. Sometimes that means that you can use an already-existing protein to solve a new problem, or tweak it a bit to suit your needs, use organisms as a platform to produce something, or all of them at the same time!
Analogous to how we’re starting to treat cancer in a personalized manner in humans, Syocin is growing plant-based bactericides that protect AND CURE fruit and vegetable crops in a non-toxic way AND highly-precise way.
They’re developing a synthetic biology platform to design and build bactericidal proteins in months instead of years, targeting specific bacteria instead of all the phytomicrobiome, and starting trials with a large citrus grower.
For the record, the total economic impact of bacterial infections, only on the Florida citrus industry between 2006 and 2021 was estimated to be $4.64 billion in lost revenue, 162,200 lost jobs, and $1.76 billion in lost tax revenue—big problem to solve.
New Organs
YC’s slogan and best piece of advice for founders is: make something people want. It should make sense but it turns out that lots of startups just don’t follow this logic.
Most bio startups know what people want. Even the startups that are tackling ecological problems, are doing so because these problems end up affecting us, humans. It’s the tech that needs to work.
Now, I’ll tell you what’s something that lots of people want but few are keen on providing: organs.
Only 15% of the organ demand was covered in 2018 (according to the WHO), leaving more than 800,000 people waiting around the world. That means that, only in the US, 17 people die each day waiting for an organ transplant.
On the lower side of the spectrum of solutions, we have something like an app that connects potential donors to potential receivers. On the right-most side is growing new organs all-together. The first only solves the channelling problem but not the demand problem, and the second will take at least 1 more decade to be feasible.
Who’s gonna fill the demand in these years before we figure out feasible and viable organ printing? Unsurprisingly, the answer is pigs. More specifically, CRISPRed pig organs that don’t get rejected by the human immune system and don’t infect human cells with the porcine endogenous retrovirus.
Even though monkeys are more genetically similar to humans, pigs are a more ideal animal to work with because of our physiology (weight being an important factor). Even from a supply perspective, pigs have short gestation times (4 months) and relatively large litters (15 piglets) which could ultimately help provide more transplants for humans who need them.
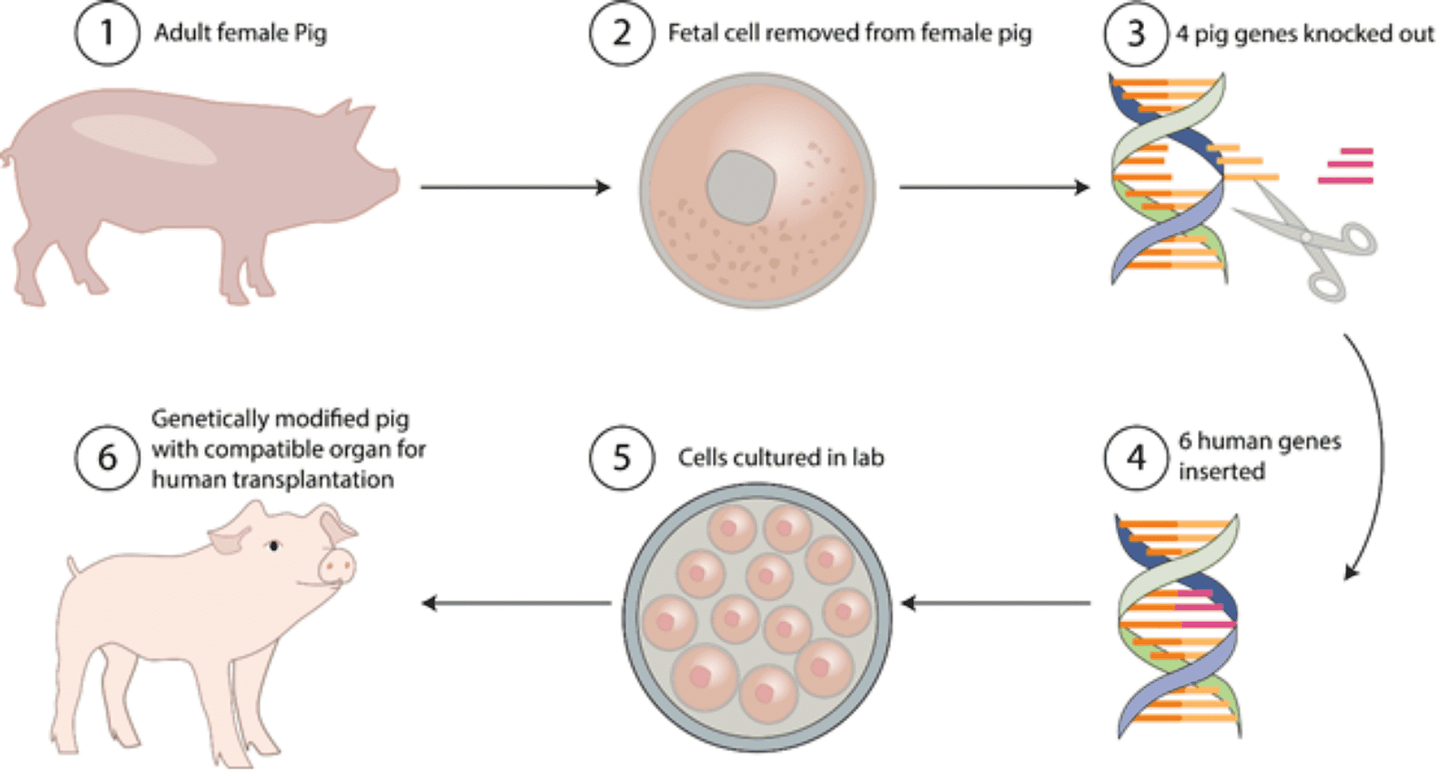
New Organs is one of the few groups in the world that has been successful in obtaining genetically modified piglets (5 of them!). Using CRISPR, they knocked out two genes: one is the growth hormone receptor so the pigs wouldn’t have a very altered weight and the second one is the GGTA1 protein so the pigs don’t have glycosylations on the surface of their cells that can be rejected by the human body.
Their next step is to produce one more colony of genetically modified pigs!
BreakPET
“Reduce, reuse, recycle” - I got told since kindergarten. I didn’t know until now though, that, of the 40 million tons of plastic waste generated in the U.S. in 2021, only 5% to 6% was recycled.
Why? A lot of the local recycling programs I’d seen seemed to have an economic incentives: as a consumer, you get paid give your plastic to recycling centers instead of throwing them away and these centers make a profit themselves by turning waste into new products. At least that’s the case for places with relatively good waste management.
Still, recycled materials actually lose 2/3 of their economic value. Plastics made from different types of polymers melt at different temperatures and need to be separated before recycling, which is costly and time-consuming. Additionally, most recycled plastics don’t maintain their properties so they need to be combined with virgin plastic.
To solve this problem, BreakPET is doing has developed AND is already commercializing specific and thermostable enzymes that depolymeryze PET into their monomers (terephthalic acid and ethylene glycol). In simpler words, they can make virgin PET from PET waste.
Some of you might be wondering how this could have been possible for organisms to evolve enzymes that degrade PET given that this material is so (relatively) new. Others might be asking how BreakPET is actually different from many of the iGEM teams that have sought to degrade PET using bacteria…
In 2018 BreakPET found a gap. PETase enzymes were specific but not as thermostable and cutinase enzymes–which naturally degrade cutin in plants and can also degrade PET–but were not specific enough. They then decided to engineer enzymes that are both thermostable and specific, and then genetically modify bacteria to express them.
After joining GRIDX, receiving investment from them in 2020, and incorporating in Mexico and the US, BreakPET has generated more than 60 variants of enzymes, increasing thermal stability by 15 ºC.
Most importantly, they can do this at a profit, generating $450-720 in utilities per ton of recycled plastic. It’s okay to say “wohoo, let’s save the world” while we understand that not every good-doing company is a non-profit ;)
Nanotransfer
A controversial opinion, as we might remember from Steve Jobs and Henry Ford, is that people don’t know what they want. Right now, the equivalent to “faster horses” might be something like “more specific chemotherapies”.
Something I chatted about with Christina Agapakis (creative director at Ginkgo) on my podcast recently, is that it’s natural to be scared of these new technologies; many researchers themselves are quite cautious and thoughtful regarding the impact of their innovations. But we—and I really mean all of us—are all in the same boat: we just want solutions to problems.
That said, it’s clear that gene therapies are the best solution we have so far to an innumerable amount of diseases. Ever since its FDA approval in 2017, over 20,000 patients have been treated with CAR-T cell therapies. Just a few months ago this teen, who’s not much younger than me, got another chance at life (after leukemia) thanks to base editing! And c’mon, do I even need to remind ourselves that 1.4 billion doses of COVID vaccines were mRNA?!
Now, that makes the case for “why gene therapies now”. One of my favorite question to ask in (bio)tech is “what needs to be true for X to do Y?”. In this case, we need to answer: what else needs to be true for gene editing to cure billions?
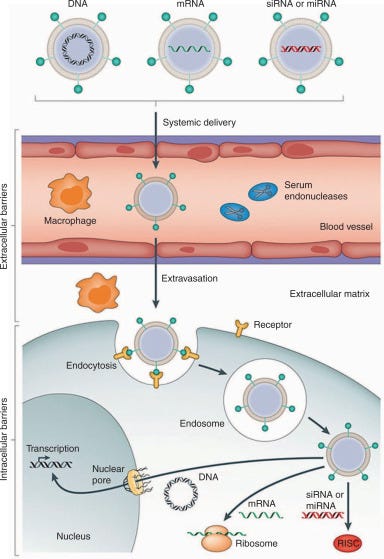
Delivery vectors, the vehicles through which gene editing systems travel, are part of this equation. Though viral vectors are currently the most common ones, they’re not eligible for use in all patients, do not allow for re-treatments, and have low tissue-specificity, can’t carry large nucleic acid sequences, and are not very versatile (if the gene or the target cell is changed, each vehicle must be synthesized from scratch).
Nanotransfer wants to help bioengineers’ have an easier time at curing disease. They’re enabling the power of gene therapies by building nanoparticles that are eligible for all patients, non-immunogenic, safe and biocompatible, with potential for re-treatment, tissue specific, have a higher payload, and are flexible for different kinds of therapies.
Naturanova
It’s not only our planet’s health that suffers from an excess of carbon. Just as climate change is a consequence of us getting excess carbon off the ground and releasing it into the air, most of the diseases we know are a consequence of excess sugar consumption; just as the world is addicted to petroleum for its efficiency as a fuel versatility for material production, our brains can’t help but fall all into the the dopamine-release-capacity of sugar.
The solutions I’ve told you about so far involve the use of super-microbes, nanotech, gene editing, and what not. It’s time to introduce plants.
What comes to mind when you hear that word? Personally, it’s either a fruit like an orange, or just a bunch of leaves. I mean, ever since the agricultural revolution, we’ve been using them produce some of our most basic goods, like clothes, shelter, and food. There’s even a millenary history of ancient cultures using plants as medicine too.
All this time, we’ve been using plants as machines and yet, we’re just starting to realize that they could be whole factories. If most of the largest genomes known to Earth are plants, there’s got to be something more to them than just fruit.
The question is: how do we go from plant to platform? Our best allies here are Artificial Intelligence and bioinformatics. With enough data and a robust workflow, we can use these tools to design new ingredients that are optimized for flavor, health, sustainability, and cost.
Naturanova is building just this. As synthetic biology company at heart, they following the Design-Build-Test (DBT) cycle:
Design: they use bioinformatics and AI to understand how proteins are involved in flavor perception and design protein candidates.
Build: they test these proteins in the lab
Test: after selecting the ideal protein, they use microorganisms to create a sugar substitute that is non-caloric, digestible, pH stable, has no aftertaste, and is high intensity ;)
Nat4Bio
1/3 of the food we produce worldwide gets wasted or lost. The difference is important, since most developed countries waste food (after reaching final consumer) while most developing countries waste food due to lack of infrastructure.
Not only does this impact farmers and companies in terms of profit; the FAO estimates that food loss and waste 1account for 8-10% of global greenhouse gas emissions (GHGs). Even those of us who have access to a fridge could have our health affected too by consuming products with synthetic substances used to extend shelf life.
Food going rot is something that most of us take for granted, something that is “just the way it is”. In principle, it’s only either oxidation, photodegradation, or microorganisms eating the food and leaving waste products that make food stink.
This said, it is ironic yet wonderful how much Nat4Bio does what it stands for: instead of letting microbes spoil food, they’re fermenting them (fungi and bacteria) to produce the world’s first edible coating for fresh lemons, blueberries, and avocado!
By regulating the gas exchange, especially of oxygen, between fruit and its environment, Nat4Bio can slow down microorganism growth. All of this, while keeping the coating transparent, odorless, and tasteless. The same fresh lemons we love, lasting longer:
Did I mention that, after GRIDX, Nat4Bio has been part of the world’s famous biotech accelerator, IndieBio? ;)
BSafe
I’ve told you about the inefficiencies of our current agricultural system, specifically in terms of managing pests. What I haven’t said is that global warming and the rise of atmospheric CO2 levels is only exacerbating this problem, expanding the geographic range, overwintering survival, and number of generations of pests.
Just as Syocin, BSafe is tackling this problem through smart biotechnology. In contrast to Syocin, BSafe is using RNAi molecules to target specific DNA sequences in pests, to selectively limit their growth and expansion.
Essentially, a biotechnology like RNAi works by slicing up RNA so it doesn’t translate into a protein. In contrast to a gene editing tool like CRISPR, this biotechnology can prevent protein expression without altering the genome. Again, it’s like targeting only specific cancer cells to prevent them from multiplying, except it’s for pests.
Delivery methods for things like this can be quite interesting. Going from nanoparticles like those Nanotransfer is developing, to viruses or even incorporating the system into the organism itself as a “synthetic immune system”. The chosen approach really depends on the technical efficacy and labeling the regulations around different methods.
[Insert your bio startup]
Coming back to the article that has sparked this collaboration with GRIDX, I have some new thoughts on biotech startups in LatAm and around the world.
While I once complained about LatAm not being so moonshoty, I no longer think that you need to reinvent the wheel to be a great entrepreneur. For innovation can be about building completely novel technologies, perhaps a more important question to ask is “how many people am I impacting?”.
Adapting a technology to the necessities of a specific population is a bold endeavor on its own. Whether it’s xenotransplants, biopesticides, or ingredients, I can see that many GRIDX companies are building for LatAm. Part of their mission is to take these technologies into the hands of millions—an even more ambitious quest.
Further, this does not mean that there are no 0-1 startups in LatAm or in the GRIDX portfolio. Just think about startups like argenTAG — they are going from a paper published in Nature to building 10x better genomic tools while being advised by renown figures like George Church and have gone through the IndieBio accelerator after GRIDX.
Double-clicking on that, I’m very curious to see if the number of startups in the GRIDX-IndieBio pipeline continues to grow. Again, I think this depends on who these companies are building for and what their mission is.
Specifically for editing technologies, there is a clear predominance of agriculture and climate companies. Given that we’ve been editing plants for decades and that Latin America is a strong agricultural region, this is unsurprising. It is, however, the increasing precision in our tools and the rising capability in bioinformatics and automation tools, that I’d be particularly excited about in this decade.
I don’t care if you want to build in Caracas or Greece, if you want to solve cancer for billions or make the cure for a rare disease. Whether you want to use fungi, yeast, or a cocktail of these, just know that the future won't be built by a wiz. Consider joining the bio movement please :) —human-made.
That cipher, I’m actually a bit skeptical on since other studies have found that it’s 127 million tons of CO2 (<1% of total emissions in 2018) that food waste is responsible for… but that’s another story.








Great article! There's so much happening in this sector. Who created the first illustration?